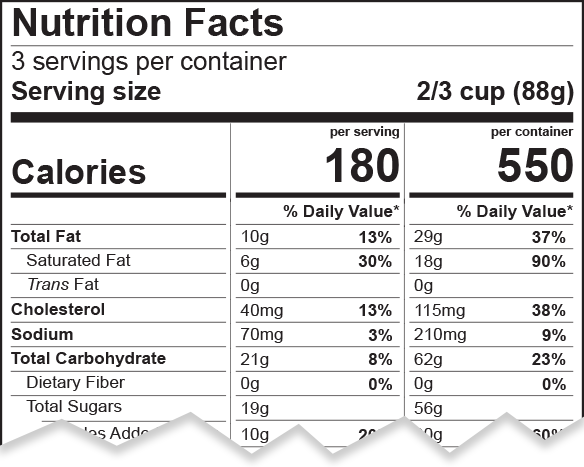
As part of the 2016 FDA labeling revisions, portions sizes that Americans tend to consume were taken into consideration and as a result, Reference Amounts Customarily Consumed (RACC) were revisited…
Tag: Labeling & Compliance

Using the Attributes Feature in Genesis R&D for Tracking BE Material and other Recipe Characteristics
The Attributes feature in Genesis R&D Foods was designed to help you track specific properties in your Ingredients and Recipes in order to comply with mandatory labeling laws for bioengineered…

Dietary Fiber on the Nutrition Facts Label
Editor’s note: this post was originally published on June 27, 2016, and has been updated for accuracy and comprehensiveness. The final rule for FDA’s 2016 updates to Nutrition Facts labeling…

Labeling Spices and Spice Blends
When labeling spices and spice blends, it’s important to consider a variety of factors, including what is considered a spice, and how to treat spices in a recipe vs. as…

Using a PDCAAS Value to Display %DV Protein on Your Supplements Facts Label
Protein is one of the three macronutrients that make up the bulk of the human diet and is found in a variety of plant and animal sources. But not all…

CFIA Sugars-based Ingredients Definition and How to Comply Using Genesis R&D
Sugars-based ingredients in the Ingredient Statement In order to help Canadians easily identify hidden sources of sugar, the Canadian Food Inspection Agency (CFIA) Food Labelling regulations require that sugars-based ingredients…