There are many reasons why clear ingredient statements and allergen declarations are important. For one, consumers want to know what is contained in the food they’re consuming. Creating clear ingredient…
Tag: Labeling & Compliance

Simplify Label Compliance with Genesis Foods
Food product labels play a crucial role in conveying vital information about your products and brand. Consumers rely on these labels to gain insights into the ingredients, nutritional value, and…

How Genesis Foods Can Accelerate Accurate Nutrient Calculations
As a food and beverage manufacturer, it’s important for your product labels to not only include accurate ingredient information, but that they also include accurate nutrient values. For consumers, reviewing…
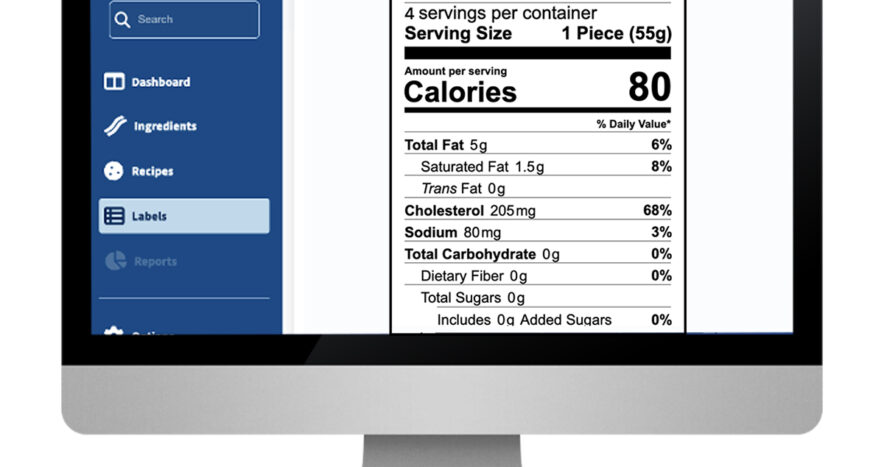
Trustwell Launches the Next Generation of Genesis Foods
As we recently announced, Trustwell is excited to introduce a new iteration of its flagship product to food and beverage industry leaders. Genesis Foods is a SaaS solution built on…

The Benefits of Front of Package Labeling for Consumers and Manufacturers
Consumers are accustomed to checking the Nutrition Facts panel on the back or side of a packaged food item for information about ingredients, allergens, and nutrition. In recent years, calls…

Demystifying Nutrition Facts Labeling: How to Navigate Different Package Sizes
In the United States, you can find a variety of Nutrition Facts labels on food packaging. The Food and Drug Administration (FDA) (and in some cases, the US Department of…

