In an effort to clear up some confusion with the Menu Labeling regulations that go into effect May 7, 2018, the FDA has released a Supplemental Draft Guidance Document. FDA…
Author: Stephanie Ulrich

Yield Options in the ESHA Database: Choosing Cooked vs Raw
Customers often ask the question, “How do I enter the correct weight of meat when my recipe refers to a beginning raw weight, but my nutritionals need to show cooked…

PHOs Are No Longer Considered GRAS Here's How We Can Help
Both the US FDA and Health Canada are working towards reducing trans fats from the food supply by banning the use of partially hydrogenated oils (PHOs). For companies with a…

How to Use the Audit Trail Feature in Genesis R&D Food
The Audit Trail feature in Genesis R&D records and reports changes made to Ingredients, Recipes, Food Menus, and Advanced Labels. This is especially helpful in the record-keeping of formulations and…
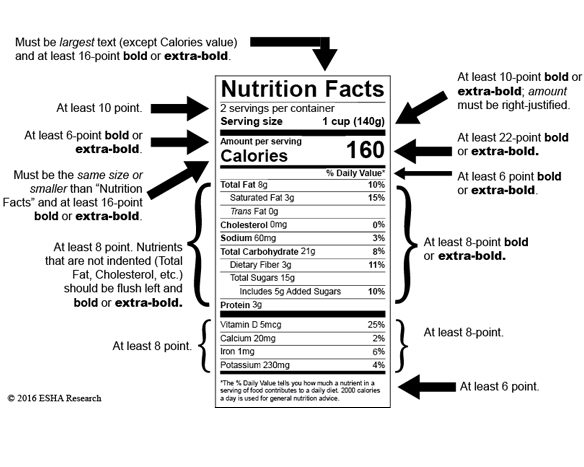
FDA Intends to Extend Nutrition Facts Labeling Compliance Date
The FDA announced their intent to extend the compliance dates for Supplement and Nutrition Facts Labeling. At this time, the FDA has not commented on when the new enforcement dates will be…

Genesis R&D Supplements 1.2 Release
The latest version of Genesis R&D Supplement Formulation and Labeling software is now available and brings with it many important enhancements. While all of the changes and additions are listed in…

