Genesis R&D Food Version 11.4 New Features Overview
Software program: Genesis R&D Foods
This release of Genesis R&D Foods incorporates many exciting new features. For an extensive list of changes and updates, please see the release notes.
We covered each of these updates in detail in our recent webinar, Genesis R&D 11.4 Overview.
For this tutorial, we will focus on the most important enhancements:
Document Attachments
Genesis R&D now gives you the option to attach documents to your Recipe, Ingredient, Advanced Label, or Food Menu. All file types can be attached to a record including PDF, JPG, CSV, MP4, and more.
Check out the document attachment tutorial and attachment use-cases here.
2016 Nutrient Content Claims
Nutrient Content Claims for the new 2016 Nutrition Facts label are now available. The software will evaluate Nutrient Content Claims according to the % DVs associated with the 1990 or 2016 label category.
See also our Claim Feature tutorial.
Shortened Label
The new shortened label option allows users to move nutrients with insignificant amounts to the footnote. When the “Show Insignificant Footnote” option is selected, the following nutrients will be moved to the footnote (if they qualify) Cholesterol, Dietary Fiber, Total Sugar, Added Sugar, Vitamin D, Calcium, Iron, Potassium, and Trans Fat.
See also our Footnote tutorial.

Users can choose to show the nutrient value, even when insignificant, by navigating to Nutrient Options and selecting “Show even if insignificant.” (Note in the example above, Vitamin D is shown even though its amount is insignificant.)
Small Package Options
When working with small packages, space can be limited. When creating tabular and linear labels, users now have two more options for saving space:
- The option to hide the %DV footnote
- The option to hide quantitative values and just show the %DV
See also our Tabular and Linear labels tutorial.
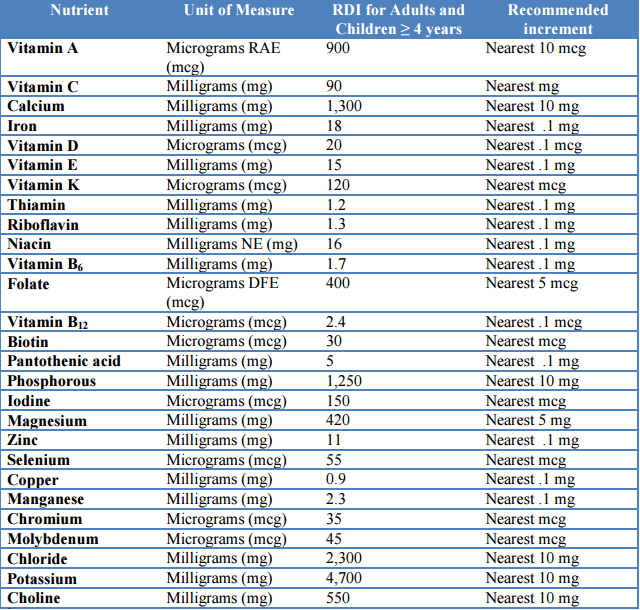
Applying Draft Guidance Rounding Rules
You can choose to apply rounding rules to your label per the new Draft Guidance from the FDA.
Note: If you choose this option, it will override ALL OTHER ROUNDING RULES you may have previously selected.
To use the Draft Guidance Rounding Rules:
- Click Edit Label
- Select Format Options
- Check Use FDA Guidance for nutrient rounding
The rounding rules are summarized in this chart:
DataLink [Now Available!]
The new DataLink add-on module allows users to connect their ingredient data from their TraceGains supplier portal with ingredients in the Genesis R&D database.![]()
When your Supplier’s ingredient information is updated in TraceGains, the DataLink module will recognize that within Genesis R&D. Users can then review the side-by-side comparison and accept (or skip) changes. When changes are accepted, ingredient records are then updated with the most current nutrition information in Genesis R&D.
