On Feb. 27, 2014, the FDA announced proposed updates to the labeling regulations for foods and dietary supplements, which Michelle Obama unveiled on the anniversary of her Let’s Move program.
Exactly 2 years and 3 months later, on May 27, 2016, the FDA published the final rules to the Federal Register.
On Jan. 1, 2020 (3 years, 7 months, 5 days after the final rules were published to the federal register), the regulations officially went into effect for food manufacturers with $10 million or more in annual food sales.
5 years, 10 months, and 5 days.
From the time the FDA announced the proposed updates to the Nutrition and Supplement facts label in 2014 to the first compliance date of Jan. 1, 2020, it took 5 years, 10 months, and 5 days.
And A LOT happened in those 5+ years. Let’s take a look.
May 2016
On May 3, 2016, the FDA sent out a news release: FDA Modernizes Nutrition Facts Label for Packaged Foods and on May 27, 2016, the FDA published 2 documents to the Federal Register.
Food Labeling: Revision of the Nutrition and Supplement Facts Labels
February 2018
On February 27, 2018, FDA published several Guidance for Industry Documents on dietary fiber and other key issues related to the new Nutrition Facts labeling laws. We provided a recap of these guidance documents in a blog post.
Guidance for Industry: Proper Labeling of Honey and Honey Products
May 2018
On May 3, 2018, the FDA issued its final rule to extend the compliance dates for new Supplement and Nutrition Facts Labels.
“The compliance date for the Nutrition Facts and Supplement Facts label final rule and the Serving Size final rule has been extended from July 26, 2018, to Jan. 1, 2020, for manufacturers with $10 million or more in annual food sales. Manufacturers with less than $10 million in annual food sales receive an extra year to comply—until Jan. 1, 2021.”
June 2018
On June 20, 2018, the FDA published a Guidance for Industry document to “identify for manufacturers specific, additional isolated or synthetic non-digestible carbohydrates that we intend to propose adding to the list of those that meet our regulatory definition of dietary fiber.”
March 2019
On March 27, 2019, the FDA announced their intent to propose that “cross-linked phosphorylated RS4 — regardless of source — be added to the definition of dietary fiber.”
Constitute Update: FDA Grants Citizen Petition for Dietary Fiber
For more information on labeling Dietary Fiber, check out our Dietary Fiber blog posts on our website.
June 18, 2019
On June 18, 2019, the FDA finalized the draft Guidance for Industry document (originally published in February of 2018) relating to labeling added sugars.
August 2019
In August of 2019, the FDA published two more Guidance for Industry documents.
*For a condensed overview for Converting Units of Measure, check out our Nutrient Unit Conversion Calculations blog post and our Nutrient Unit Conversion Calculations Cheat Sheet.
Guidance for Industry: Policy Related to Cranberry Products with Added Flavorings
December 2019
In December of 2019, just days before the compliance date, the FDA issued one final Guidance for Industry document and posted a revised update for another.
January 2020
The deadline for compliance has passed for food manufacturers who make $10 million or more in annual sales, but the FDA has granted a six-month grace period.
“… FDA plans to work cooperatively with manufacturers to meet the new Nutrition Facts label requirements and will not focus on enforcement actions regarding these requirements during that time.’
This extends the compliance enforcement date to July 1, 2020. The deadline for manufacturers with less than $10 million in annual sales remains the same — Jan. 1, 2021.
In the meantime, we encourage you to reach out to us if you need help. We have a team of labeling consultants who can walk you through the final steps of compliance or review your labels if you have any questions.
As always, stay tuned to this blog for any updates and remember that our eLearning Center remains a helpful resource for a host of compliance issues.
Other posts you might be interested in
View All Posts
Product Formulation
5 min read
| August 12, 2016
FAQ: Why Am I Not Seeing Dietary Fiber on My Label?
Read More
Food Labeling
2 min read
| January 4, 2019
FDA Revisions to the 2016 Nutrition Labeling Regulations
Read More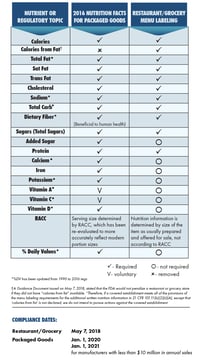
Food Labeling
3 min read
| March 15, 2019

