In the U.S., Omega Fatty Acids are not included on the list of mandatory label nutrients or voluntary label nutrients for food products and do not have an associated RDI or DRV. Therefore, they are not allowed to be listed within the Nutrition Facts label. (Note: Supplement Facts labels and packaging are subject to different regulations. Therefore, you will often see Omega-3 and Omega-6 listed within the Supplement Facts label.)
However, the FDA does allow manufacturers to declare the presence of Omega Fatty Acids on the packaging itself, outside the Nutrition Facts label.
In this blog, we will cover:
- The requirements for declaring Omega Fatty Acids
- Examples of Permissible Packaging Statements
- Examples of Impermissible Packaging Statements
- How to Use Genesis R&D to Track Omega Fatty Acids
Requirements for Declaring Omega Fatty Acids
When declaring the presence of Omega Fatty Acids, make sure your statement meets these regulatory requirements:
- The information listed must be truthful and not misleading.
- The declaration must include the quantitative amount per serving (may be expressed either as the number of milligrams or grams per serving).
- The declaration does not characterize the level of the nutrient in the product (there are a few exceptions for Alpha-Linolenic Acid Nutrient Content Claims).
- In some cases, certain qualified health claims for eicosapentaenoic acid (EPA) and docosahexaenoic (DHA) omega-3 fatty acids can be made.
Examples of Permissible Omega Fatty Acid Statements
The following statements are examples of what you could declare on your package. Remember, you can use grams or milligrams.
- X grams of Omega-3 Fatty Acids per serving
- X grams of Omega-3 Fatty Acids per serving from Y (state the source food or ingredient)
- Contains X grams of Omega-3 Fatty Acids per serving
- Provides X grams of Omega-3 Fatty Acids per serving
These statements would be permitted because they are truthful and not misleading, include a quantitative amount per serving, and do not characterize the level of the nutrient in the product.
Examples of Impermissible Omega Fatty Acid Statements
The following statements would not be permitted on a product package:
- Contains omega-3 fatty acids
- Provides omega-3 fatty acids
These statements would not be permitted because they are missing the quantitative amount.
- Good source of omega-3 fatty acids
- High in omega-3 fatty acids
These statements would not be permitted because nutrients that do not have an established DV may not include characterization statements.
Don’t forget… If you make a statement of fact such as “Contains 250 milligrams of Omega-3 Fatty Acids per serving” on the front-of-pack, this is enforced as a nutrient content claim. As a result, if your product exceeds threshold levels of total fat, saturated fat, cholesterol, or sodium, you would be required to include a disclosure statement (refer to 21 CFR 101.13(h) for more information about disclosure statements).
Using Genesis R&D Foods to Report Omega-3 and Omega-6
Genesis R&D includes fields for tracking Omega-3, Omega-6, as well as other individual fatty acids. When you enter the fatty acid data for your Ingredients, Genesis R&D will automatically calculate the per serving amounts at the Recipe level.
Steps for Entering and Reporting Nutrient Data
- Review your Nutrients to View settings to make sure Omega-3, Omega-6, and other nutrients you wish to track are available in your ingredient entry screen and reports.
- Enter nutrient values at the ingredient level as reported on your spec sheet.
- Review the Spreadsheet or Multi-Column reports to make sure:
- there is no missing data and
- you see the total per-serving amount of the nutrients
- Use the amount to generate the proper packaging declaration.
Using Nutrients to View Tutorial

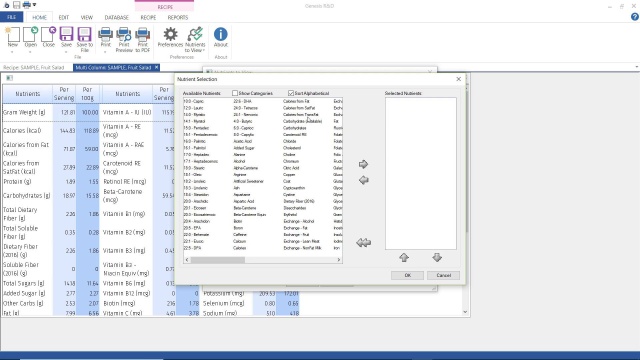
4:22
Please note, this blog post is intended as a broad overview and should not be construed as legal advice on any specific facts or circumstances. Please be sure to consult the regulations for additional requirements.
Tag(s):
Other posts you might be interested in
View All Posts
Product Formulation
20 min read
| September 28, 2021
Using a PDCAAS Value to Display %DV Protein on Your Supplements Facts Label
Read More
Food Labeling
3 min read
| July 19, 2017
PHOs Are No Longer Considered GRAS Here’s How We Can Help
Read More
Food Labeling
2 min read
| April 23, 2021

