In the U.S., food products are regulated by two separate federal agencies: the Food and Drug Administration (FDA), which is part of the Department of Health and Human Services (HHS), and Food Safety and Inspection Service (FSIS), under the jurisdiction of the U.S. Department of Agriculture (USDA). Both agencies have guidelines for labeling food products, which can be confusing when your food products may fall under both agency umbrellas.
So, let’s break down what foods are covered by each agency, and the key differences between the FDA’s labeling requirements and the FSIS’s requirements for Nutrition Facts tables.
FSIS vs FDA: What foods do they regulate?
The USDA’s FSIS has the authority to enforce regulations on food safety, packaging, and the labeling of meat, poultry, and processed (liquid, unshelled) egg products. Their primary goal is to “protect consumers from misbranded and economically adulterated meat, poultry, and egg products” through truthful labeling and the use of “Best if Used by” date labels.
However, there are a few inclusions and exclusions when it comes to the FSIS’s jurisdiction:
- Although fish is under the FDA’s jurisdiction, FSIS is responsible for inland and coastal catfish that belong to the order Siluriformes.
- Products that contain 3% or less of raw livestock meat or poultry meat, or 2% or less of cooked red or poultry meat, are under the jurisdiction of the FDA.
- Closed-face (ready-to-serve) meat and poultry sandwiches fall under FDA’s rules, while open-faced sandwiches are covered by USDA FSIS regulation.
As for other foods, the FDA covers everything else, which amounts to about 75% of the food supply in the U.S. This includes, namely:
- Milk and dairy products
- Shell eggs or other egg products not covered by FSIS
- Game meats
- Seafood (except Siluriformes catfish)
- Fruits and vegetables, both raw and processed
- Processed foods with 3% or less of raw and 2% or less of cooked livestock meat and poultry
- Infant formulas, dietary supplements, and other processed foods
What is required for an FSIS Nutrition Facts label?
According to the FSIS’s Basics of Labeling, the FSIS requires a Nutrition Facts Label for three food product categories:
- Processed meat and poultry products.
- Major cuts of single-ingredient raw meat or poultry products. There are about 35 major cuts for meat and 10 for poultry.
- Single-ingredient raw meat and poultry products that are sold as ground or chopped, whether they’re major cuts or not.
A few important things to note about these three categories:
- Non-major cuts – for example, beef flank steak or chicken tenders – will require a Nutrition Facts Label if they are sold as ground or chopped but will be exempt if they are sold whole.
- Small businesses are exempt from the Nutrition Facts Label requirement for non-major cuts sold as ground or chopped. However, they must label any major cuts.
- For labeling purposes, sausage products and hamburgers are not classified as ground or chopped meat, therefore they don’t qualify for the small business exemption.
- Normally, packers are the ones required to provide the Nutrition Facts Label because they produce the final product.
In December 2016, FSIS announced that it would amend the Nutrition Facts Label regulations for meat and poultry products to reflect recent scientific research and dietary recommendations, along the same lines as the FDA’s update in the same year. However, the update hasn’t been finalized yet for FSIS, whereas the FDA has already finalized their changes.
Until the updated rules are finalized, the USDA allows for the adoption of either the 2016 FDA format or the 1990 current USDA format. However, you will need to follow the rules established for each label in full, including calculating nutrients, formatting choices, and determining Daily Values (DVs). Once the ruling is finalized by the USDA, companies will need to comply with the final rule by the effective date.
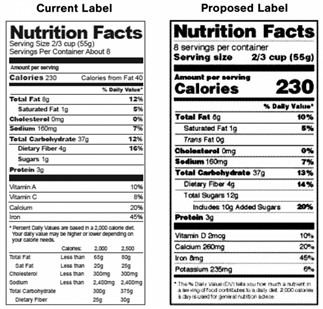
There are some key differences between the 1990 current FSIS label and the 2016 updated FDA label, so let’s break those down for clarity.
Formatting changes
1990: The font sizes are smaller and more uniform.
2016: The font sizes for Serving Size, Calories, and Nutrition Facts are larger than the other line items. Additionally, some values are bolded, like Protein, Cholesterol, Total Fat, Sodium, and Total Carbohydrates.
In 2021, FSIS released a document that explains the font differences with examples of allowed alternatives. We also offer a blog post that breaks down the major formatting differences with the 2016 FDA label.
Calories from fat, saturated fat, and trans fat
1990: The calories from fat must be declared in addition to the total number of calories, while calories from saturated fat may be included on a voluntary basis. As for trans fat, the content doesn’t need to be declared, and content of stearic acid, polyunsaturated fat, and monounsaturated fat may be declared voluntarily.
2016: In the FDA format, there’s no requirement to indicate calories from fat separately from total fat. However, it is mandatory to indicate the content of saturated and trans fat on the label.
Added sugars
1990: Added sugars is included in total sugars and doesn’t need to be declared separately.
2016: In the FDA format added sugars must be declared in addition to total sugars.
Declared vitamins and minerals
1990: Vitamin A, Vitamin C, Calcium, and Iron must be declared.
2016: The FDA now requires Vitamin D, Calcium, Iron, and Potassium mandatory for declaration, while other vitamins and minerals can be declared on a voluntary basis.
Daily Values (DVs)
1990: Use the recommended Daily Values established in the same year.
2016: Use the updated percent Daily Values (% DVs) established in 2016 when determining nutrient contents. (Check out the FDA’s Quick Reference Guide for a list of some of the changed values.)
RACCs
Once it is finalized, the new version of the USDA FSIS label will also update the reference amounts customarily consumed per eating occasion (also known as RACCs).
However, until the update is finalized, you will have to use the old, 1990 RACCs for meat and poultry products, even if you decide to use the updated label format. The FDA currently does not have RACCs for meat and poultry products (as those technically fall outside of their jurisdiction), so the only RACCs available are those provided by the USDA.
(Note: For single-ingredient meat and poultry products, RACC is 4 oz for raw meat and 3 oz for cooked meat. RACCs for processed poultry and meat products are listed in the Federal Register.)
Where can I find more information on FSIS labeling?
The USDA’s website is full of referenceable materials to help both consumers and food manufacturers understand labeling requirements, including a full list of labeling policies.
But, if you’re finding yourself a little overwhelmed by regulations, we also have a team of labeling superstars that can lend a hand. To learn more about our compliance team’s services and costs, complete our contact form and a consulting representative will reach out as soon as possible.
Tag(s):
Other posts you might be interested in
View All Posts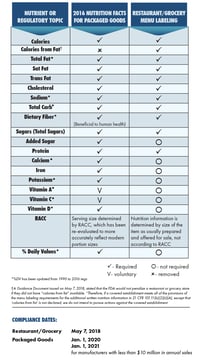
Food Labeling
3 min read
| March 15, 2019
FDA Regulations: Menu Labeling vs Food Labeling
Read More
Food Labeling
2 min read
| January 4, 2019
FDA Revisions to the 2016 Nutrition Labeling Regulations
Read More
8 min read
| November 28, 2022

